DiscoverCT
Clinical trials are an important part of medical innovation and our public health infrastructure. These trials, which include interventional and observational studies of human subjects, generally evaluate the efficacy and safety of drugs, devices, and other interventions in order to increase medical knowledge.
The National Institutes of Health (NIH) have maintained a publicly accessible registry of observational and interventional trials since 1997, when the Food and Drug Administration Modernization Act (FDAMA) mandated registration of all Phase II-IV clinical trials. This database has become more widely used since 2005, when all major scientific journals instituted a requirement that a trial be registered in the database prior to first patient being enrolled in order to publish results. This makes the database the main source of clinical trial information worldwide.
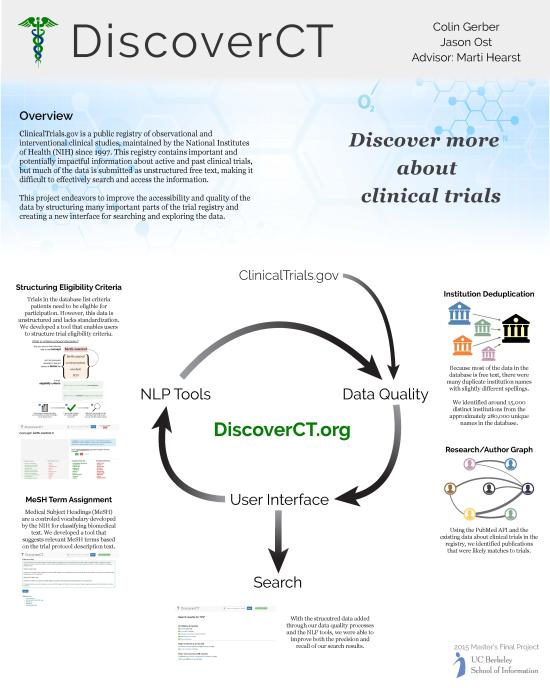
Despite the public availability of this data and its importance to safety and innovation, few efforts have been made to analyze and present the information in a way that benefits patients or researchers.
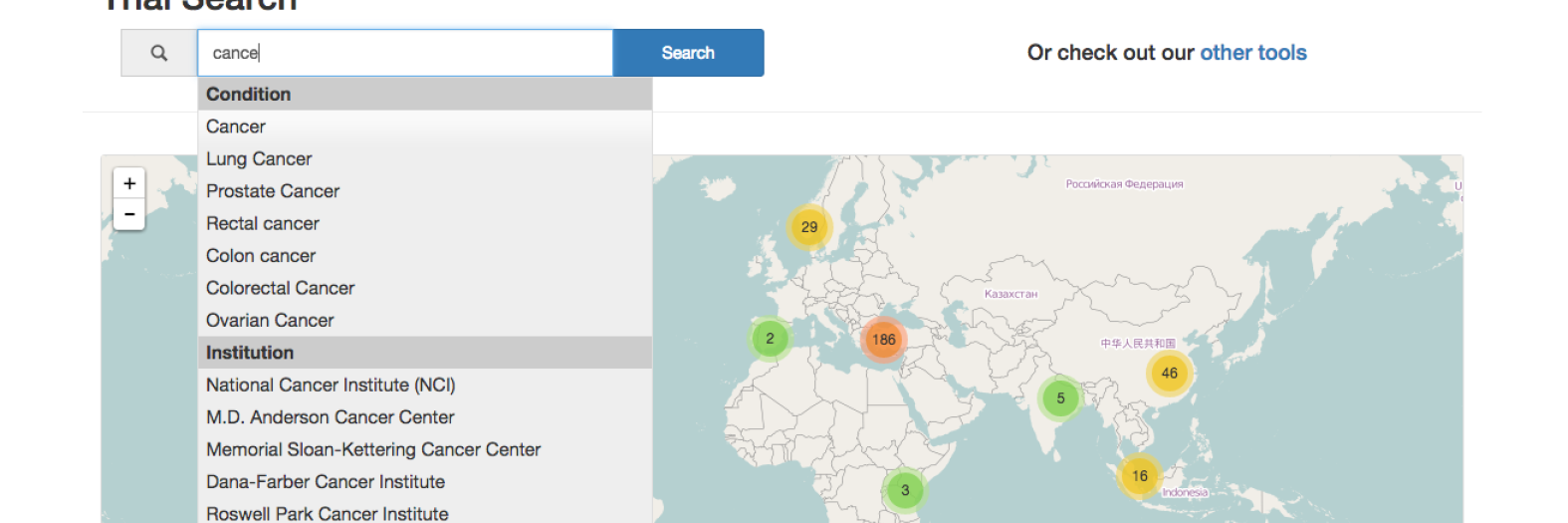
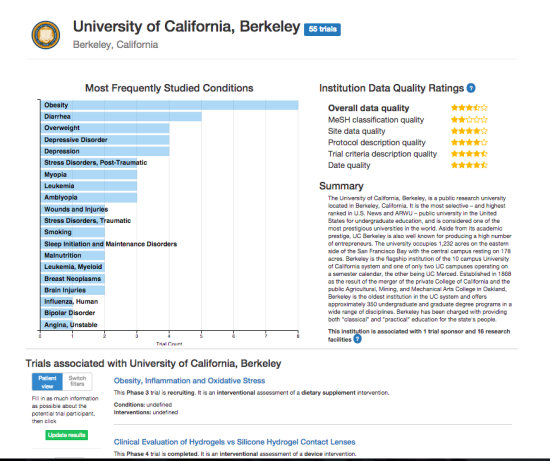
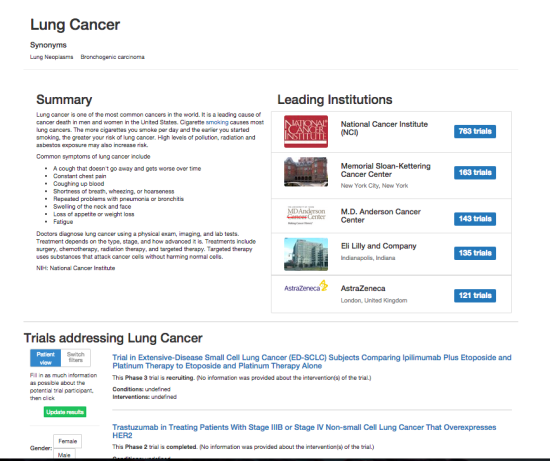
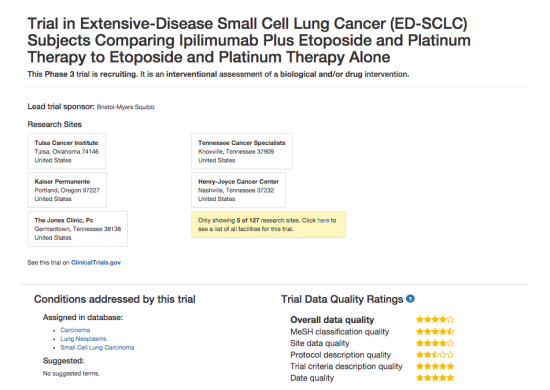
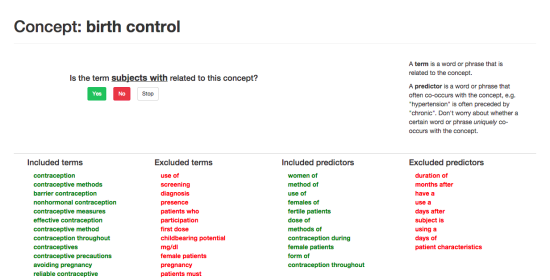
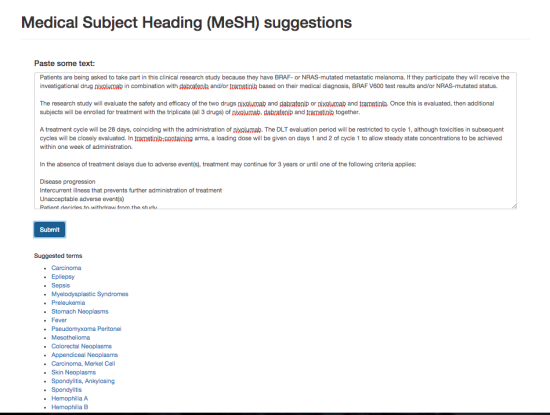
DiscoverCT addresses this shortfall by providing a user-friendly search interface to find trials related to a particular condition or institution, as well as offering tools to help trial investigators and other interested parties enhance the existing data in order to improve information retrieval via the search interface. Specifically, DiscoverCT has a tool that allows users to define eligibility criteria concepts (e.g., “tobacco use”) so that trials may be filtered by these concepts, as well as a tool that permits users to assign additional Medical Subject Heading (MeSH) terms that describe the condition(s) addressed by a specific trial.